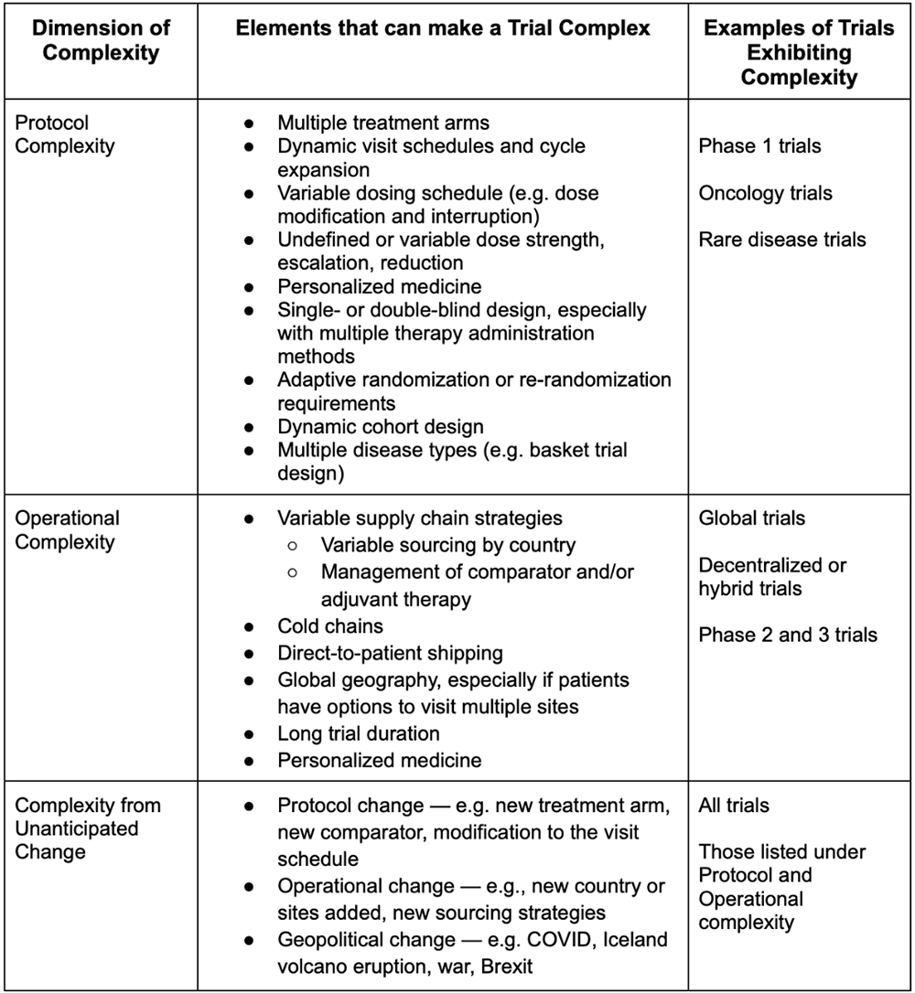
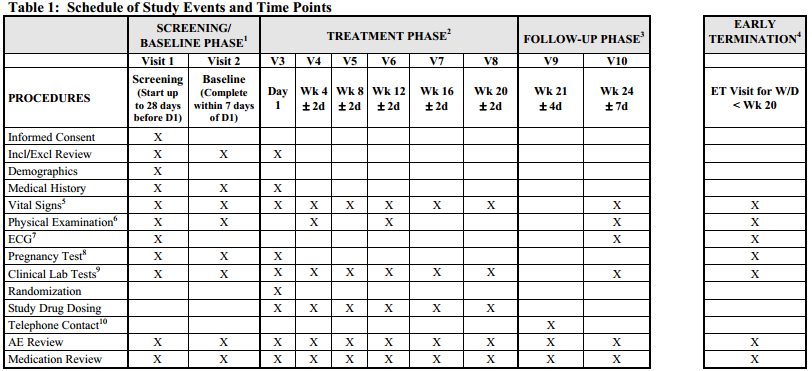
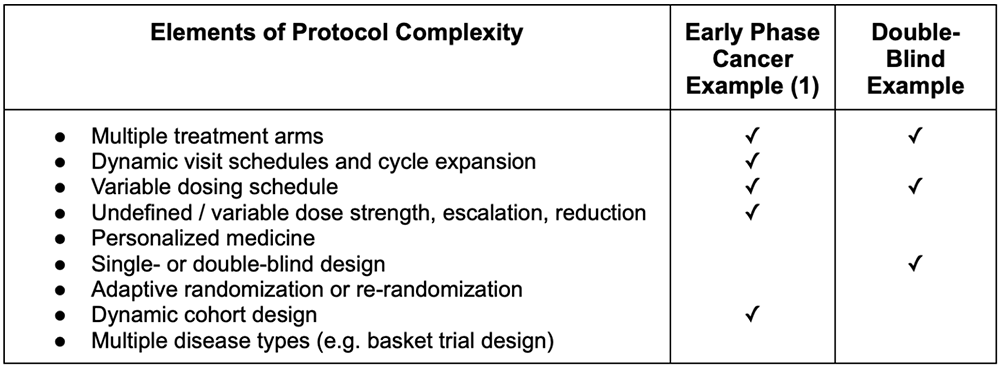
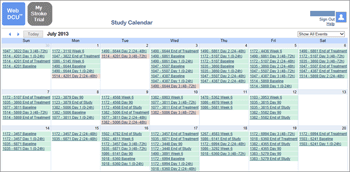
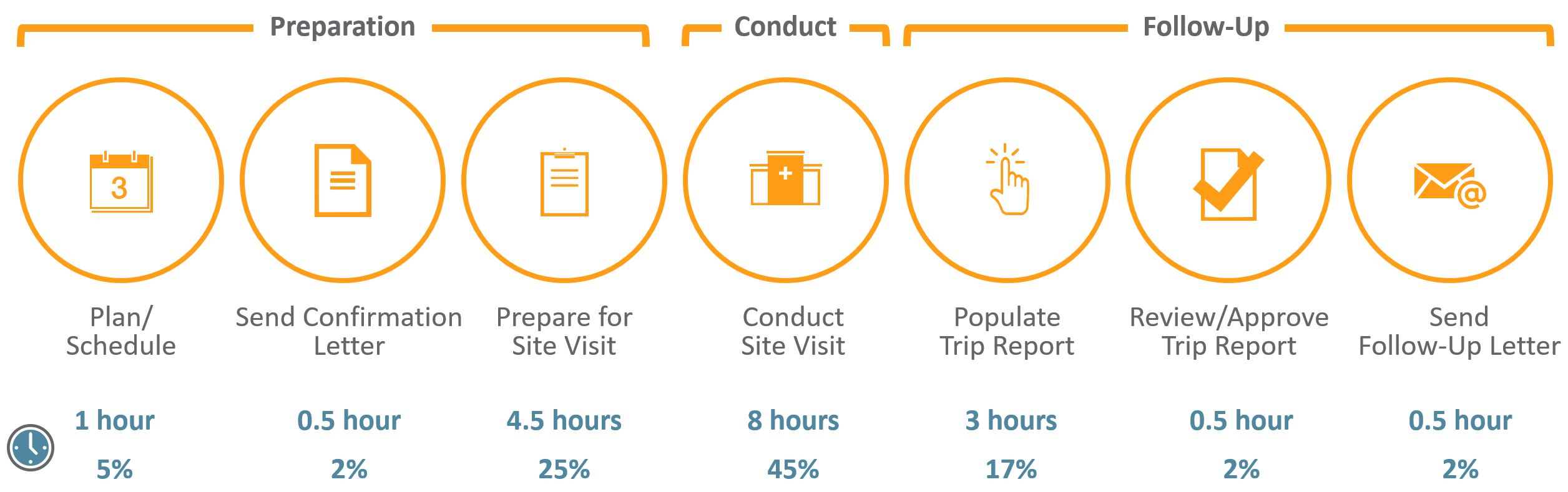
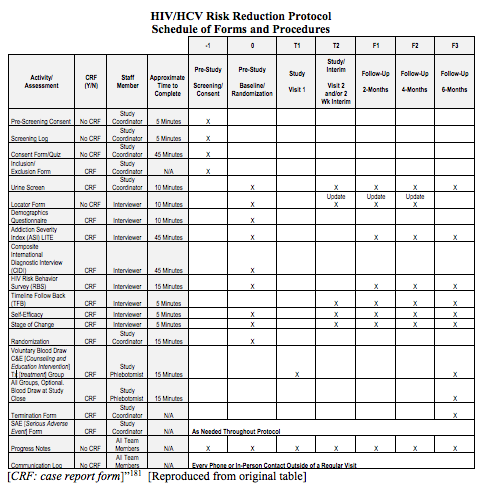
David Cloutier Director, Research Center Management and Development Budgeting for Industry Sponsored Clinical Trials. - ppt download

Schedule of the clinical trial. We randomly allocated the 80 patients... | Download Scientific Diagram
Therapeutics for Dengue: Recommendations for Design and Conduct of Early-Phase Clinical Trials | PLOS Neglected Tropical Diseases

Table 1 from PCR adjusted cure rates in clinical trials of antimalarial drugs in Africa : Influence of extended follow-up and consecutive day blood sampling | Semantic Scholar

Figure 1 from Knowledge-data integration for temporal reasoning in a clinical trial system | Semantic Scholar