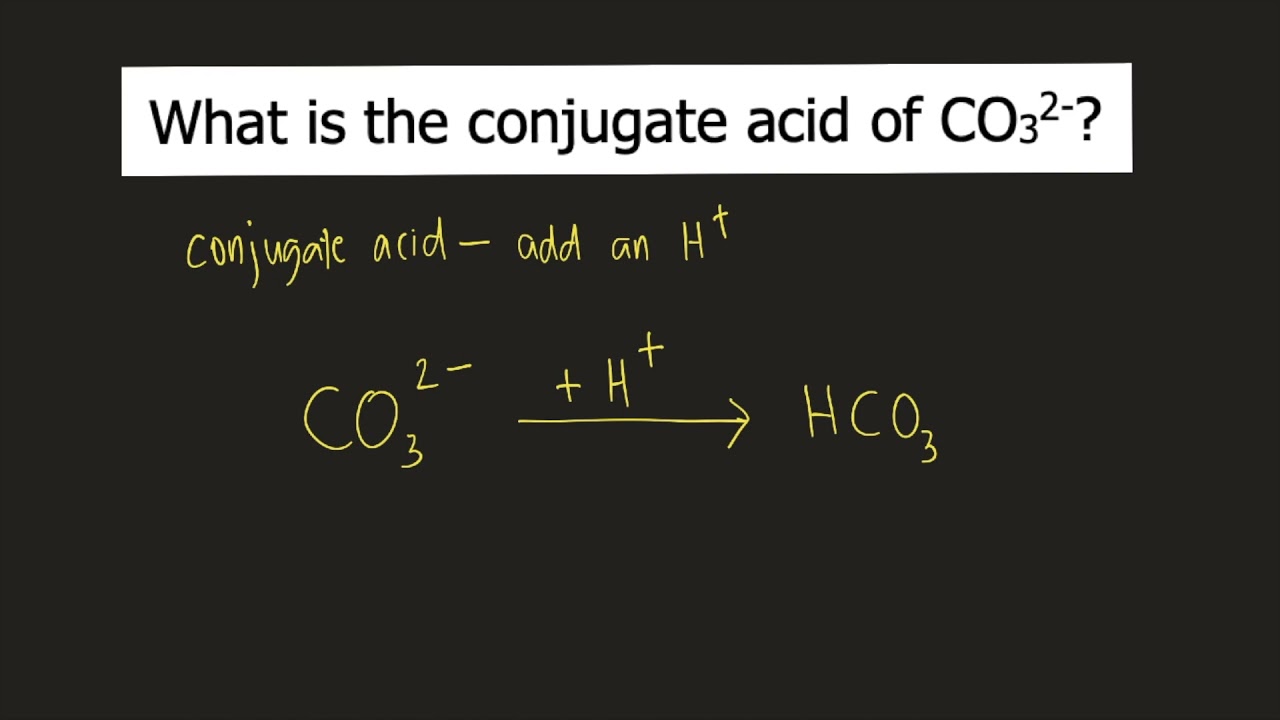
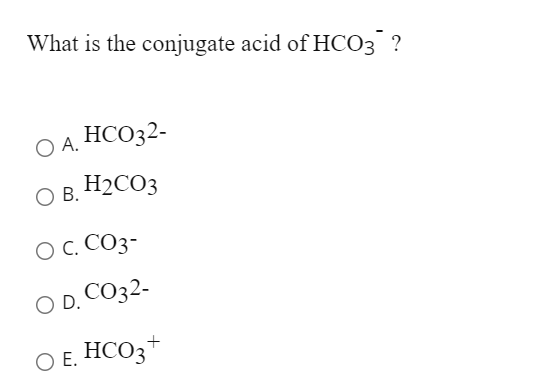Chemistry Help: The carbonate ion (CO3 2-) acts as a Bronsted base with water and equations - YouTube

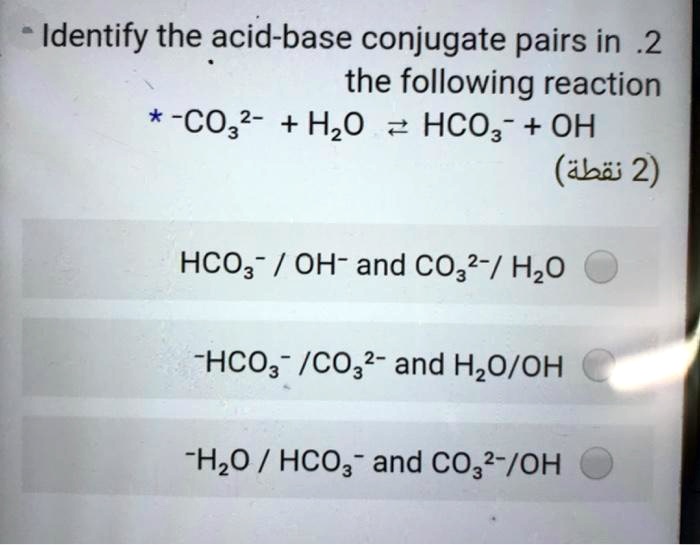
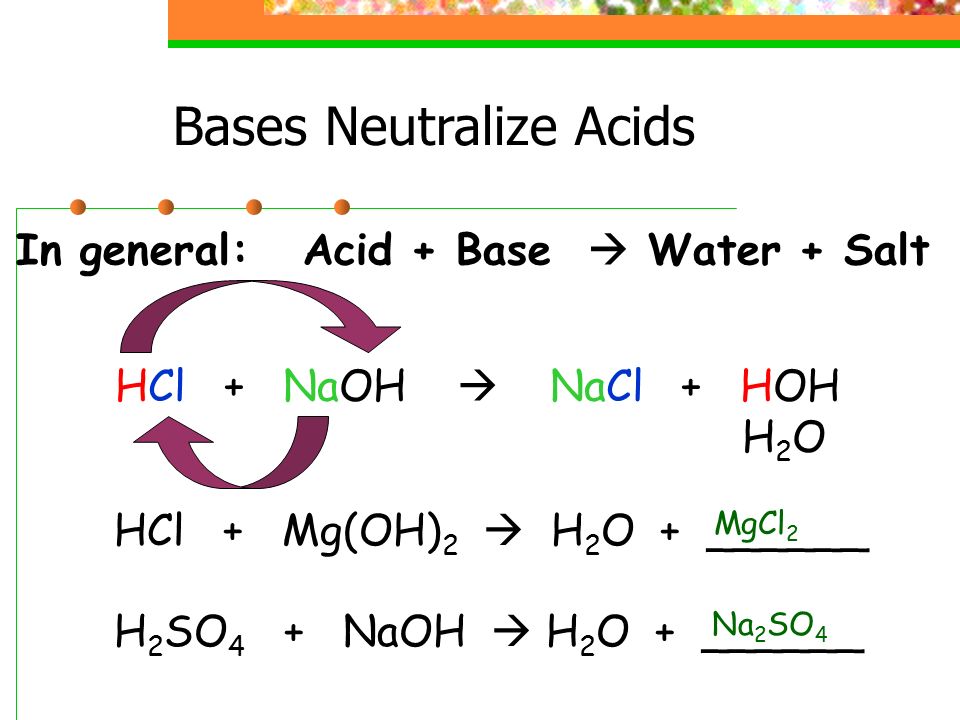
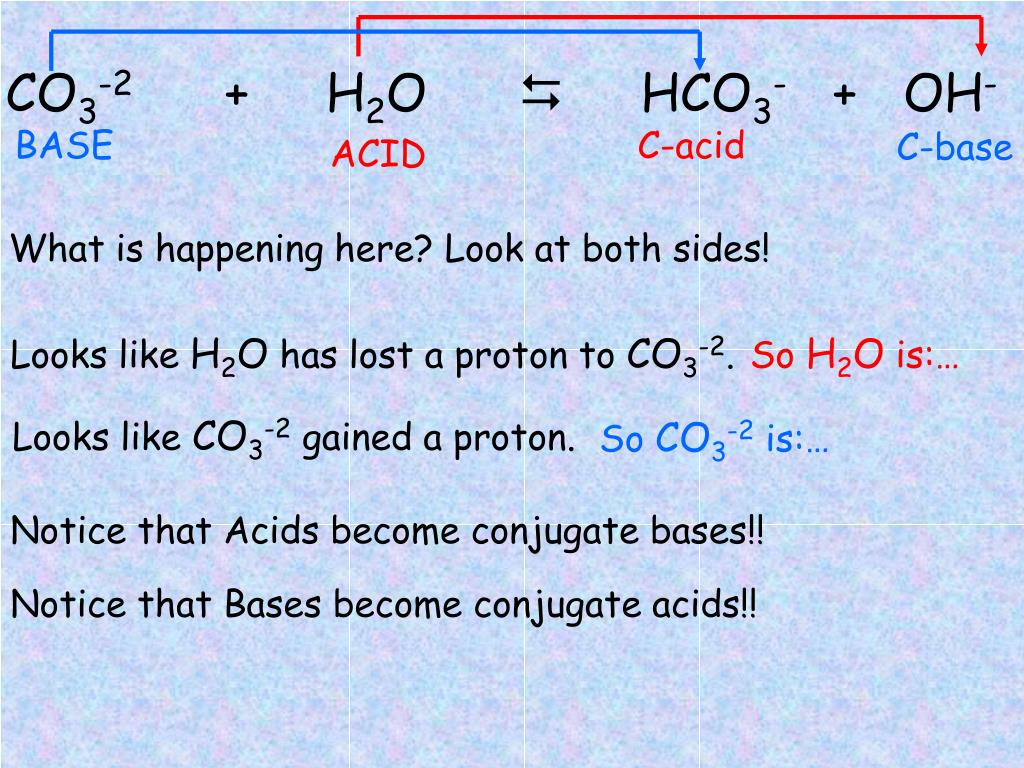
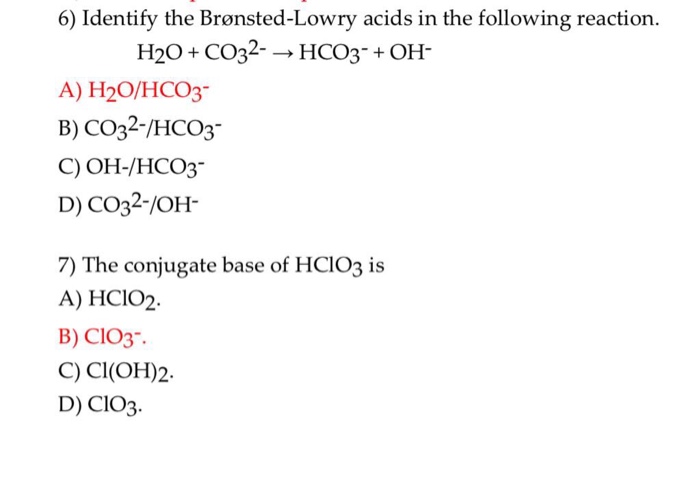
CO3^2- (aq) + H2O (l) \rightarrow (HCO3)- (aq) + OH- (aq) Identify the reactant that is a Bronsted- Lowry Acid and the reactant that is a Bronsted- Lowry Base in the reaction.
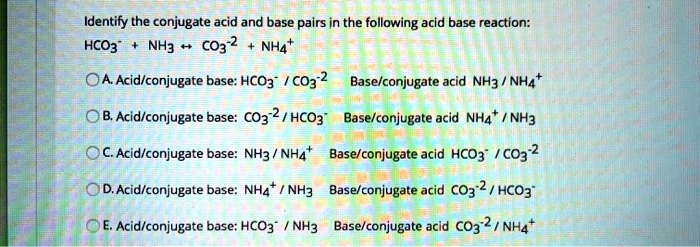
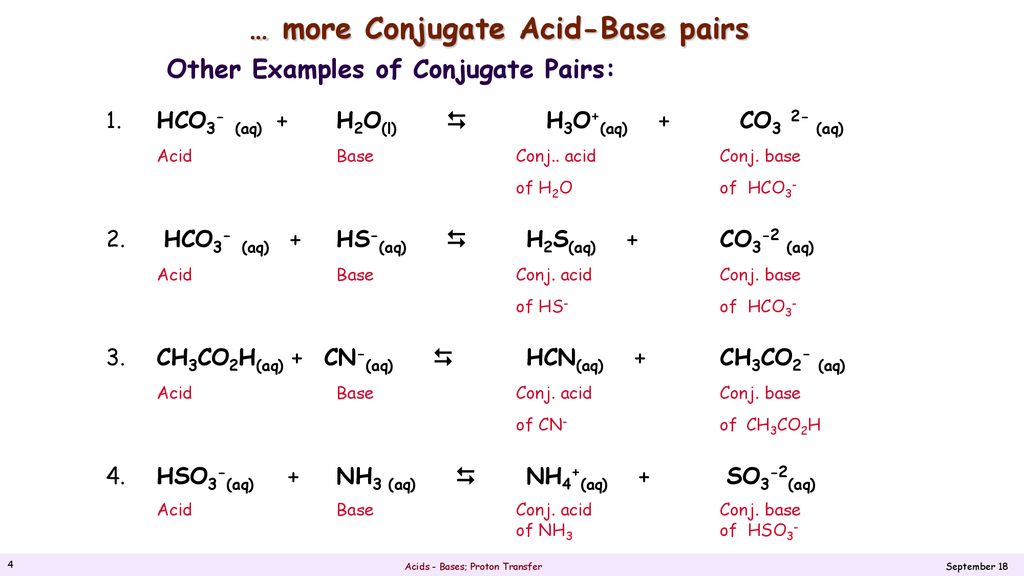
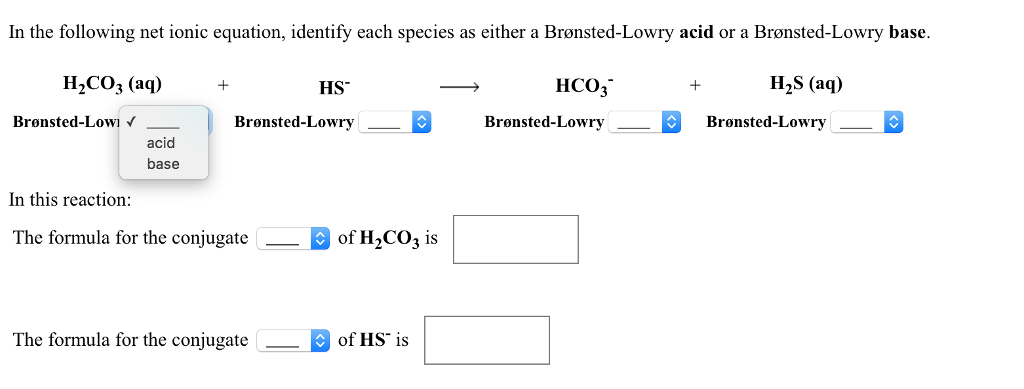
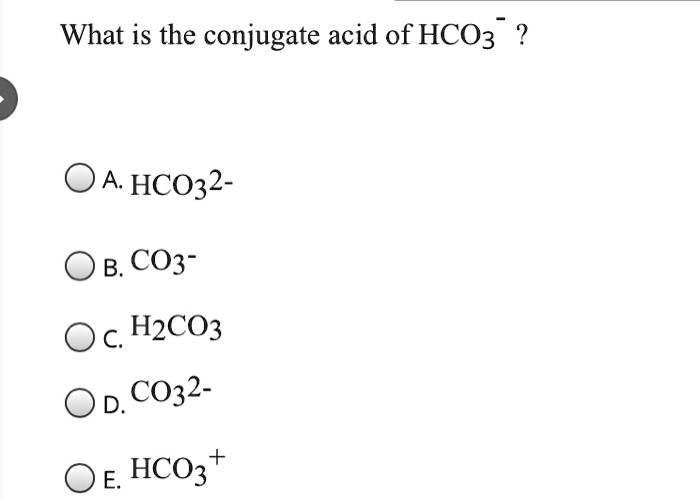
SOLVED: Identify the conjugate acid and base pairs in the following acid base reaction: HCO3 NH3 CO3-2 NH4t Acidlconjugate base: HCO3 CO3-2 Baselconjugate acid NH3 NH4 B Acidlconjugate base: CO3-2 / HCO3"

The Importance of Acid–Base Equilibria in Bicarbonate Electrolytes for CO2 Electrochemical Reduction and CO Reoxidation Studied on Au(hkl) Electrodes | Langmuir